

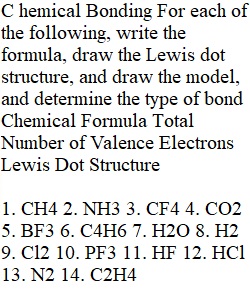
Q C hemical Bonding For each of the following, write the formula, draw the Lewis dot structure, and draw the model, and determine the type of bond Chemical Formula Total Number of Valence Electrons Lewis Dot Structure 1. CH4 2. NH3 3. CF4 4. CO2 5. BF3 6. C4H6 7. H2O 8. H2 9. Cl2 10. PF3 11. HF 12. HCl 13. N2 14. C2H4
View Related Questions